The patients
Patients who underwent minimally invasive colorectal surgery from March 2018 to December 2018 at Samsung Medical Center, Seoul, Korea were selected as subjects for this study. Minimally invasive surgery included conventional laparoscopy, single-incision laparoscopic surgery, and robotic surgery. The inclusion criteria were as follows: patients over 19 years of age, without clinically significant abnormal findings on preoperative examination, who had undergone minimally invasive surgery to produce an incision length of 3 to 6 cm, and who could use patient-controlled analgesia (PCA). Patients with hypersensitivity (or history of hypersensitivity) to ropivacaine or other amide-type local anesthetic agents, those who have undergone emergency local surgery or septic disease, those who suffered from mental illness, those who had taken medications and painkillers within a month before surgery, and those with serious comorbidities such as cirrhosis of the liver, renal failure or cardiomyopathy, were excluded. This study was approved by the Institutional Review Board (IRB) of Samsung Medical Center (IRB No.2018-01-109), and written informed consent was obtained from all subjects participating in the trial. This study was performed in accordance with all applicable guidelines and regulations. The trial was registered with the Clinical Research Information Service (CRIS) (cris.nih.go.kr; KCT0006079, Hee Cheol Kim, 03/03/2021). This study did not receive research funding from the Department of Health and Welfare, so there was no requirement to register with CRIS prior to the study. Following the recommendations, we registered the clinical study in the CRIS after the start of the study.
Surgical design
This was an open, parallel, randomized, non-inferiority clinical study. Descriptions of the Gel and On-Q system, as well as application techniques, have been previously reported19. Application of Gel and On-Q was performed during surgery, in the process of performing intra-abdominal surgery and suturing the wound. The gel pack consisted of a pre-filled syringe of gel, a syringe connector and an empty syringe for ropivacaine (Fig. 5). Initially, the empty syringe was filled with 3 ml of 0.75% ropivacaine, then this syringe was connected to the syringe prefilled with gel using the connector. Then 6 ml of a mixture of gel and ropivacaine was easily obtained by repeatedly pushing on each side of the syringe. For the experimental group, a ropivacaine hydrogel based on poloxamer 407 was applied between the peritoneum and the fascia after mixing 0.75% ropivacaine (3 ml, total dose of 22.5 mg) with 6 ml of the gel which constituted the product (Fig. 6).
Gel pack consisting of gel, connector and empty syringe for ropivacaine.
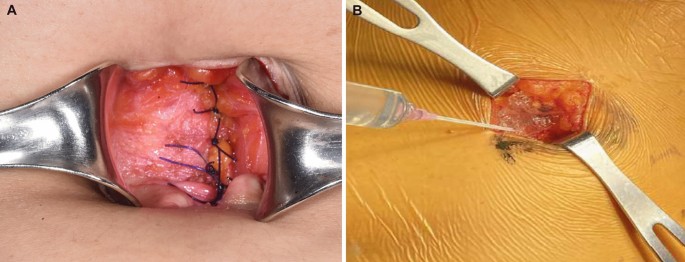
Application of gel to the wound site.
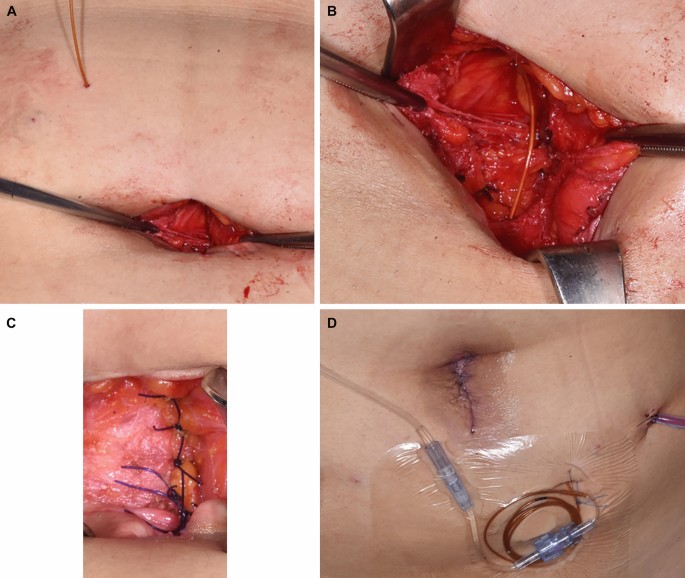
For the comparator group, the On-Q system consisted of a 25 mm silver-coated 19 catheter and a continuous PCA pump. For the control group, the catheter was inserted through a 6.5 cm catheter between the peritoneum and the fascia and connected to an injection pump containing 0.2% ropivacaine (96 mL [flow rate: 2 mL/hour] total dose of 192 mg) (Fig. 7).
Immediately after surgery, both groups received concomitant intravenous PCA (30 mL [1500 mcg] of fentanyl and 70 ml of normal saline), which was delivered via a manual infusion pump (model M1015M, Woo Young Medical Co. Ltd., Chungbuk, Republic of Korea). The total PCA volume was 100 mL, the rate was 1.0 mL/hour, the bolus volume was 1.0 mL, and the lock time was set at 15 min. In cases where the patient complained of pain (NRS score ≤ 4), rescue medication (pethidine 25 or 50 mg) was authorized up to 72 hours postoperatively.
The patients were allocated either to the experimental group (Gel) or to the active comparator group (On-Q) according to a randomization table. Randomization (in a 1:1 ratio) was done to reduce bias that may arise in patient allocation and to increase the comparability of the two groups. Block randomization was performed by generating random numbers with four decimal places per person using the Random (RAND) function in Microsoft Excel (Microsoft Excel 2019 MSO 16.0.10389.20033). Each assignment envelope was sealed and returned to the investigator. Subject numbers were assigned by the clinical laboratory and the envelope was opened by the clinical trial coordinator in charge of the study. Patients and investigators were unaware of treatment allowances.
Data gathering
The amount of fentanyl PCA used, the amount of rescue analgesic (pethidine) used, and the pain score (NRS; scale of 0 to 10, with 0 representing no pain and 10 the worst pain imaginable) were assessed at 6, 24, 48, and 72 hours postoperatively. Blood pressure, pulse, body temperature, complete blood count, prothrombin time, activated partial thromboplastin time and serum laboratory data (including glucose, blood urea nitrogen, total protein, albumin, bilirubin, aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase) were measured at 48 h, 72 h and 8 days ± 4 days after surgery to capture the occurrence of any adverse effects.
Primary and secondary outcomes
The primary endpoint was the average total consumption of fentanyl used in IV-PCA up to 72 hours after surgery in the two treatment groups. Secondary endpoints were the amount of rescue analgesia (pethidine) used and pain scores (NRS) at 6, 24, 48 and 72 h postoperatively. The occurrence of adverse events was monitored during the admission period. Vital signs and laboratory test data were collected and evaluated for safety outcomes.
Security endpoint
During the admission period, careful monitoring for adverse events was carried out. Vital signs data and laboratory test results described above were collected and analyzed to assess safety.
statistical analyzes
The study was designed to determine non-inferiority for the primary outcome based on the non-inferiority assumption20. According to previous studies3,17,21, the maximum mean difference in fentanyl PCA use over 72 h between the Gel and On-Q treatment groups was 409.96 mcg and the mean difference in the total amount of fentanyl PCA used up to 72 h post-treatment. operation between the two groups was 394.80 mcg. The non-inferiority margin (197.4 mcg) was conservatively set at half of 394.80 mcg. Based on this value, it was calculated that 62 patients were needed, including a dropout rate of 10%, with a type 1 error of 2.5% and a power of 80%.
This study used SPSS for Windows version 27.0 (SPSS, Chicago, IL, USA) for all analyses. Chi-square test, Fisher’s exact test, independent two-sample t-test, or Mann-Whitney U-test (when the normality assumption was not satisfied) were used to analyze the differences between the two. treatment groups. For multiple comparisons, analysis was performed by repeated measures analysis of variance (ANOVA) and a post-test was confirmed for the point-to-point comparison. The two-group effect test was not statistically significant, so the independent two-sample t-test was also performed to compare the two groups at each time point, and the level of significance was corrected using Bonferroni’s correction method. The results were considered statistically significant when the p-the value was less than 0.05.
Research involving human and/or animal participants
This study was approved by the Institutional Review Board (IRB) of Samsung Medical Center (IRB No.2018-01-109), and written informed consent was obtained from all subjects participating in the trial. The trial was registered before patient recruitment (cris.nih.go.kr; KCT0006079, Hee Cheol Kim, 03/03/2021).
Informed consent
This study was approved by the Institutional Review Board (IRB) of Samsung Medical Center (IRB No.2018-01-109), and written informed consent was obtained from all subjects participating in the trial.

